Although myeloablative fludarabine/busulfan (FluBu4) has been widely adopted in clinical practice, considerable interpatient variability exists in systemic busulfan exposure (AUC) when using body weight or body surface area based-dosing, leading to decreased efficacy (i.e. relapse) or increased toxicity (i.e., mucositis, veno-occlusive disease). This well-defined dose-exposure-outcome relationship has led to the widespread clinical implementation of therapeutic drug monitoring (TDM). However, individualized TDM can be time and labor intensive as well as potentially biased due to the lack of incorporation of any previously established PK data (Bayesian prior). In contrast, Bayesian maximum a posteriori (MAP) PK models consider the Bayesian prior and individualized TDM to generate a revised probability distribution (Bayes conditional posterior) to more accurately and rapidly estimate the AUC with reduced bias. There are a paucity of data comparing busulfan AUCs using individualized PK versus MAP-Bayesian-based models in adults although these more sophisticated approaches may assist in optimizing dosing of busulfan in this vulnerable population.
This was a retrospective, single-center study of patients who received FluBu4 with busulfan TDM between January 1999 and September 2019. 109 patients diagnosed with a hematologic malignancy who received either sequential (n=46) or concurrent (n=63) FluBu4 were analyzed. The median age was 48 (range: 18-66), and were Hispanic (n=38), White (n=46) or African-American (n=14). TDM was performed either after a test dose of 0.8 mg/kg (n=52) or after the first dose (3.2 mg/kg) of busulfan administered during the preparative regimen (n=71), with dosing was based on actual or adjusted body weight. For PK analysis, plasma busulfan concentrations were analyzed via gas chromatography with mass selective detection. Individualized PK data were generated using WinNonlin while the MAP-Bayesian approach utilized the Bayesian prior developed from McCune et al (Clin Cancer Res, 2014). An AUC of 4800 µM˖min/24 hours was targeted based on previous literature.
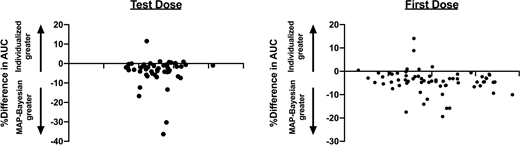
Based on individualized PK data, total recommended busulfan doses ranged from 9.3-21.3 mg/kg (-27.1% to +66.7% compared to FDA labeled dose of 12.8 mg/kg). When first-dose busulfan PK was compared between busulfan given sequentially versus concurrently with fludarabine there was a trend towards a higher AUC with concomitant administration (4651 vs. 4988 µM˖min; p=0.13). A strong correlation between the AUC generated from both the individualized PK an MAP-Bayesian models was observed with both the test dose (R2=0.91) and first dose (R2=0.86) of busulfan. Using the MAP Bayesian model, AUC predictions were on average higher (mean AUC 5069 versus 4886 µM˖min, p<0.0001) compared to the patient-specific individualized PK estimates. Figure 1 shows the Bland Altman plots for comparison of the individualized AUC vs. MAP-Bayesian estimates for test dose and first dose.
Our individualized busulfan PK approach generated relatively similar AUC values compared to MAP-Bayesian estimates, although the higher AUC generated via MAP-Bayesian predictions may allow for lower doses of busulfan to be administered thereby potentially reducing toxicity while maintaining efficacy. Further, use of the MAP-Bayesian method may allow for more rapid dose optimization and a decreased number of serum concentrations. Further prospective studies including more patients are warranted to confirm these findings.
Calip:Flatiron Health: Current Employment. Patel:Janssen: Consultancy; Amgen: Consultancy; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal